Valid molecular dynamics simulations of human hemoglobin require a surprisingly large box size
Krystel El Hage, Florent Hédin, Prashant K Gupta, Markus Meuwly, Martin Karplus
eLife 2018;7:e35560 DOI: 10.7554/eLife.35560
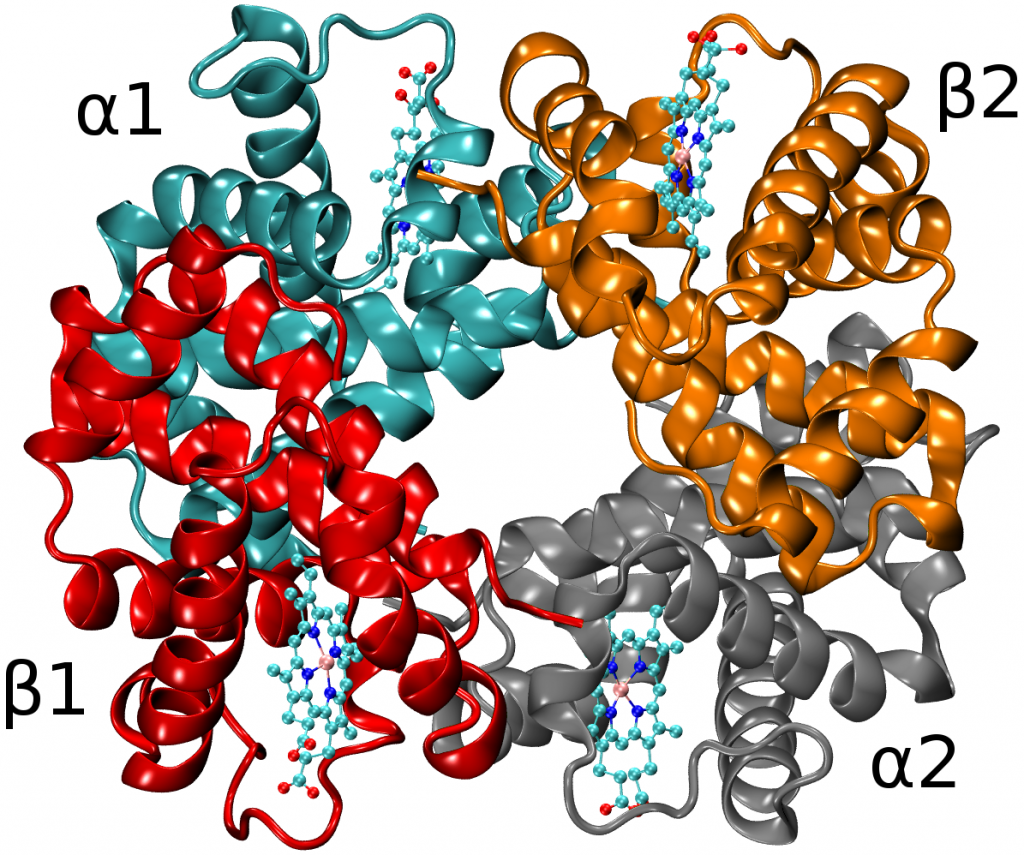
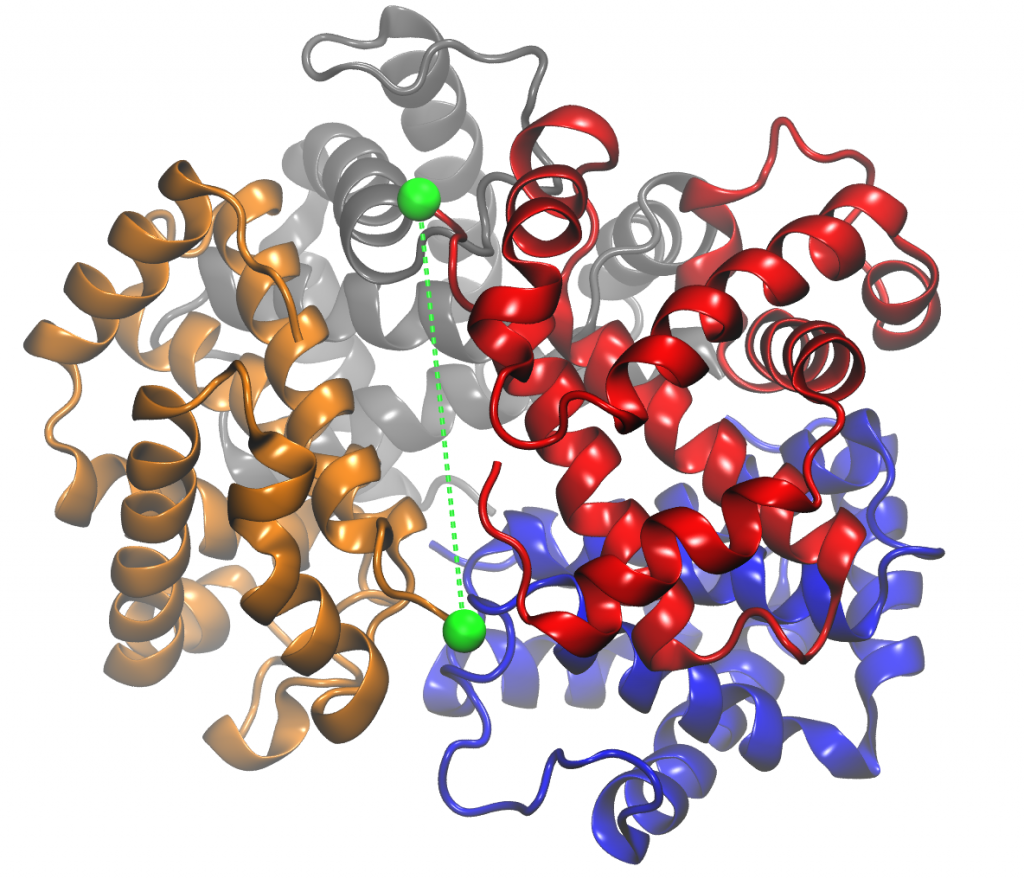
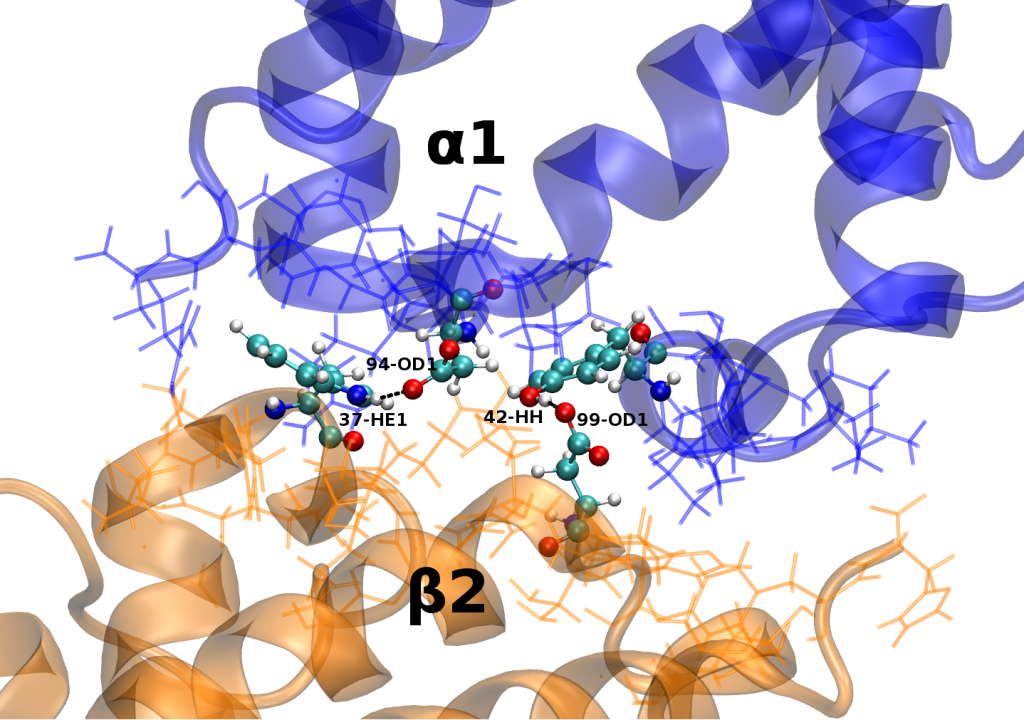
Recent molecular dynamics (MD) simulations of human hemoglobin (Hb) give results in disagreement with experiment. Although it is known that the unliganded (T0) and liganded (R4) tetramers are stable in solution, the published MD simulations of T0 undergo a rapid quaternary transition to an R-like structure. We show that T0 is stable only when the periodic solvent box contains ten times more water molecules than the standard size for such simulations. The results suggest that such a large box is required for the hydrophobic effect, which stabilizes the T0 tetramer, to be manifested. Even in the largest box, T0 is not stable unless His146 is protonated, providing an atomistic validation of the Perutz model. The possibility that extra large boxes are required to obtain meaningful results will have to be considered in evaluating existing and future simulations of a wide range of systems.